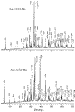
.gif)
National Institute for Research in Inorganic Materials (NIRIM), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
Received February 8, 2001
Revised Manuscript Received June 18, 2001
Abstract:
AuBa2Ca2Cu3O9 (Au-1223-Ba) and
AuBa2Ca3Cu4O11 (Au-1234-Ba), two
high-Tc superconductors, were synthesized by
high-pressure/high-temperature at 6 GPa and 1250-1300 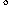 C. These phases are the third and fourth members of
the
AuBa2Can-1CunO2n+3
series. The Au-1223-Ba and Au-1234-Ba phases crystallize in an orthorhombic
primitive system with the lattice parameters a = 3.8182(4) Å, b =
3.8555(4) Å, and c = 15.445(2) Å, and a = 3.8266(3) Å, b =
3.8505(3) Å, and c = 18.494(1) Å, respectively. The Au-1234-Ba phase
showed bulk superconductivity below 99 K. The superconducting transition of the
as-grown Au-1223-Ba sample was very broad with a transition temperature of
C. These phases are the third and fourth members of
the
AuBa2Can-1CunO2n+3
series. The Au-1223-Ba and Au-1234-Ba phases crystallize in an orthorhombic
primitive system with the lattice parameters a = 3.8182(4) Å, b =
3.8555(4) Å, and c = 15.445(2) Å, and a = 3.8266(3) Å, b =
3.8505(3) Å, and c = 18.494(1) Å, respectively. The Au-1234-Ba phase
showed bulk superconductivity below 99 K. The superconducting transition of the
as-grown Au-1223-Ba sample was very broad with a transition temperature of  30 K and with a small superconducting volume
fraction. The volume fraction increased after the sample was postannealed at 300
30 K and with a small superconducting volume
fraction. The volume fraction increased after the sample was postannealed at 300
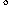 C in O2 atmosphere.
C in O2 atmosphere.
Introduction
Superconducting layered cuprates have conducting CuO2 planes separated by charge-reservoir blocks. It has been known that various metal and nonmetal elements can occupy the charge reservoir cation sites. The 5d8 Au3+ ion usually adopts square-planar coordination due to a very strong Jahn-Teller effect.1 The ionic radius of Au3+ in square-planar coordination is 0.68 Å, a value comparable to that of Cu2+ (0.57 Å).2 This seems to be the motivation of Grenoble researchers to replace copper by gold in the charge reservoir block of orthorhombic YBa2Cu3O7. Indeed, they succeeded to synthesize AuBa2(Y,Ca)Cu2O7 [Au-1212-Ba-(Y,Ca)] with Tc = 82 K at 1.8 GPa.3 The same group showed that substitution of gold for mercury in HgBa2Ca2Cu3Oy is also possible up to 40% and that the structure retained tetragonal symmetry after the substitution.4
Recently, it has been shown that high-pressure synthesis is quite effective for stabilizing cuprate layered structures with various charge-reservoir blocks.5 Since high pressure stabilizes compounds with low thermal stability at ambient pressure, gold-containing systems seem to be good candidates to be synthesized by this technique.
In the present work, we carried out phase search experiments under 6 GPa for the Au-Ba-Ca-Cu-O system, and found a new AuBa2Can-1CunO2n+3 (n = 3, 4) superconducting family, and studied the synthesized compounds by X-ray diffraction, high-resolution transmission electron microscopy, dc magnetic susceptibility, and electric resistivity measurements.
Experimental Section
BaO2, Au (99.9%), Au2O3 (86% of Au content),
CuO (99.9%), and Ca2CuO3 were used as initial reagents for
high-pressure synthesis. BaO2 was prepared by a solution route:
H2O2 and NH3 were added to an aqueous solution
of BaCl2; then the BaO2 precipitated was collected by
filtration. All these procedures were done under N2 atmosphere to
avoid contamination of CO2. After it was dried at 150 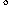 C in O2, the carbon content in
BaO2 was determined by a carbon analyzer (LECO, WR12) to be less than
0.008 wt %. Single-phase Ca2CuO3 was prepared from
CaCO3 (99.9%) and CuO at 970
C in O2, the carbon content in
BaO2 was determined by a carbon analyzer (LECO, WR12) to be less than
0.008 wt %. Single-phase Ca2CuO3 was prepared from
CaCO3 (99.9%) and CuO at 970 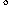 C in
air for 1 week with several intermediate grindings. Appropriate amounts of
precursors were mixed in an agate mortar and sealed into a gold capsule in a
glovebox filled with dried N2. The mixture was allowed to react in a
belt-type high-pressure apparatus at 6 GPa and 1250-1300
C in
air for 1 week with several intermediate grindings. Appropriate amounts of
precursors were mixed in an agate mortar and sealed into a gold capsule in a
glovebox filled with dried N2. The mixture was allowed to react in a
belt-type high-pressure apparatus at 6 GPa and 1250-1300 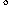 C for 2-3 h, and then was quenched to room
temperature. Very careful treatment to avoid carbon contamination was
indispensable for the present system. If BaO2 was handled in air or
commercial BaO2 was used, oxycarbonate series of phases
(Cu,C)Ba2Can-1CunO2n+36
were formed instead of the gold-based phase.
C for 2-3 h, and then was quenched to room
temperature. Very careful treatment to avoid carbon contamination was
indispensable for the present system. If BaO2 was handled in air or
commercial BaO2 was used, oxycarbonate series of phases
(Cu,C)Ba2Can-1CunO2n+36
were formed instead of the gold-based phase.
X-ray diffraction (XRD) data were collected using a powder diffractometer
(Phillips PW 1800) with Cu K radiation. Lattice constants were determined by a least-squares refinement
procedure. Electron probe microanalysis (EPMA) was performed using an analyzer
(JEOL JXA-8600MX) to determine the cation compositions of the high-pressure
phases. High-resolution transmission electron microscopy (HRTEM) observations
were carried out for some selected samples with a microscope (Hitachi H-1500)
operating at 800 kV. Magnetic susceptibilities were measured under
zero-field-cooling and field-cooling conditions in an applied field of 10 Oe
with a DC SQUID magnetometer (Quantum Design, MPMS). Electric resistivity
measurements were carried out by the conventional four-probe method with silver
paste for electrodes using a physical property measurement system (Quantum
Design, PPMS).
radiation. Lattice constants were determined by a least-squares refinement
procedure. Electron probe microanalysis (EPMA) was performed using an analyzer
(JEOL JXA-8600MX) to determine the cation compositions of the high-pressure
phases. High-resolution transmission electron microscopy (HRTEM) observations
were carried out for some selected samples with a microscope (Hitachi H-1500)
operating at 800 kV. Magnetic susceptibilities were measured under
zero-field-cooling and field-cooling conditions in an applied field of 10 Oe
with a DC SQUID magnetometer (Quantum Design, MPMS). Electric resistivity
measurements were carried out by the conventional four-probe method with silver
paste for electrodes using a physical property measurement system (Quantum
Design, PPMS).
Results and Discussion
More than 20 starting mixtures were tested varying the cation ratio and the
oxygen content. Among them, samples with starting compositions of
AuBa2Ca2Cu3O9+d
(d = 0, 0.1, 0.2) contained a new orthorhombic phase with c  15 Å (hereafter, denoted Au-1223-Ba). The d
= 0 starting mixture gave the best phase purity for Au-1223-Ba with an XRD
pattern in Figure 1. The
AuBa2Ca3Cu4O11+d
(d = 0, 0.1, 0.2) starting mixture originated a new orthorhombic phase
with c
15 Å (hereafter, denoted Au-1223-Ba). The d
= 0 starting mixture gave the best phase purity for Au-1223-Ba with an XRD
pattern in Figure 1. The
AuBa2Ca3Cu4O11+d
(d = 0, 0.1, 0.2) starting mixture originated a new orthorhombic phase
with c  18 Å (Au-1234-Ba), with the
highest phase purity for d = 0 as well (XRD pattern in Figure 1). The
lattice parameters for Au-1223-Ba and Au-1234-Ba samples with d = 0 were
a = 3.8182(4), b = 3.8555(4), and c = 15.445(2) Å, and
a = 3.8266(3), b = 3.8505(3), and c = 18.494(1) Å,
respectively.
18 Å (Au-1234-Ba), with the
highest phase purity for d = 0 as well (XRD pattern in Figure 1). The
lattice parameters for Au-1223-Ba and Au-1234-Ba samples with d = 0 were
a = 3.8182(4), b = 3.8555(4), and c = 15.445(2) Å, and
a = 3.8266(3), b = 3.8505(3), and c = 18.494(1) Å,
respectively.
Besides the main phases, both samples contained unknown phase(s) whose X-ray peaks are denoted by asterisks in Figure 1 as well as small amounts of CuO and gold metal. The presence of the impurity phases suggests that the real compositions of the orthorhombic phases may differ from the stoichiometric ones of 1:2:2:3 (Au/Ba/Ca/Cu) and 1:2:3:4. To verify this hypothesis, we carried out EPMA measurements of the Au-1223-Ba and Au-1234-Ba phases in samples with d = 0. The experimental cation ratios were determined by averaging the data of seven Au-1223-Ba grains and nine Au-1234-Ba grains. The results were 0.9(1):1.91(6):2.10(6):3.1(1) for Au-1223-Ba and 0.8(1):1.9(1):3.1(1):4.2(1) for Au-1234-Ba. In both cases the experimental ratios are close to stoichiometric values. Even though the Au-1234-Ba phase seems slightly Au-deficient and Cu-rich, it is not possible to fully confirm this because of the large experimental uncertainty. The EPMA measurements detected CuO and gold metal in both samples, consistent with their X-ray patterns. In addition, both samples contained a Cu-free unknown phase of "Ca2BaAuOy" which probably corresponds to the additional X-ray peaks denoted by asterisks in Figure 1.
The present Au-1223-Ba and Au-1234-Ba phases are the n = 3, 4 members of the homologous series AuBa2Can-1CunO2n+3, respectively. Starting mixtures with AuBa2Can-1CunO2n+3 (n = 5, 6) were tested, expecting higher-order members of Au-1245-Ba and Au-1256-Ba. Oxygen-poor 1234 compositions of AuBa2Ca3Cu4O11+d (d = -0.1 and -0.2) were also tested, since a higher-order member sometimes becomes stable under relatively low oxygen pressure.5 In addition, we extended the high-pressure reaction period up to 6 h. Despite these attempts, peaks assignable to Au-1245-Ba or Au-1256-Ba were not observed at all in XRD patterns.
The Au-1223-Ba and Au-1234-Ba phases were studied by HRTEM. Figure 2 shows electron diffraction patterns of the Au-1223-Ba phase taken along the [001], [010], [100], and [1-10] zone axes. All diffraction spots could be indexed by a primitive orthorhombic lattice consistent with the XRD patterns. No systematic extinction was observed, suggesting a space group Pmmm or Pmm2. Extremely weak additional spots corresponding to a superlattice cell with bs = 2b were sometimes observed in the electron diffraction patterns. Although the same type of superstructure was reported for the Au-1212-Ba-(Y,Ca) phase,3 the superstructure spots are much less intensive in the case of Au-1223-Ba. The electron diffraction patterns of the Au-1234-Ba phase are shown in Figure 3. The patterns are consistent with a primitive orthorhombic lattice with the space group Pmmm or Pmm2, as was the case for Au-1223-Ba. Differing from those observed in the electron diffraction patterns of Au-1223-Ba, no superlattice spots were observed for the Au-1234-Ba phase.
Figure 4 presents a HRTEM image for the Au-1223-Ba phase projected along the b-axis. The image reveals an M-1223-type structure with stacking of the planes along the c-axis, BaO-AuO-BaO-CuO2-Ca-CuO2-Ca-CuO2. In the inset of Figure 4, a schematic structure model is shown according to the lattice image. For the Au-1212-Ba-(Y,Ca) phase, it was reported that a Au-O zigzag chain is formed with doubling of the b-axis and lowering of the symmetry to P2/m from P4/mmm,3 instead of the linear one shown in Figure 4. Weak extra electron diffraction spots observed for Au-1223-Ba reveal local formation of the superstructure of bs = 2b, suggesting the formation of a similar zigzag chain in the present system. We have succeeded in obtaining single crystals of Au-1223-Ba, and single-crystal X-ray analysis is in progress to elucidate the structural details including the arrangement of the oxygen atoms in the Au-O plane.
Several
MA2Can-1CunO2n+3
[A, alkaline earth; M-12(n-1)n-A] superconducting families have been reported
for trivalent M atoms. They include Tl-12(n-1)n-Ba,7
B-12(n-1)n-Sr,8,9 Al-12(n-1)n-Sr,10 and
Ga-12(n-1)n-Sr.11-13 It is worth noting that
all these M atoms belong to the 3A elements of the periodic table and that
indium is the only element of column 3A that does not form the M-12(n-1)n-type
series. In spite of the fact that trivalent gold has a similar ionic radius to
that of indium,2 it can accommodate itself to the M-site. This fact
may be related to the square-planar coordination preference of the gold ion.
 |
Figure 4 HRTEM image for the Au-1223-Ba phase taken along the [100] zone axis. The inset represents a schematic structure model for the Au-1223-Ba phase. |
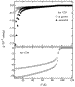
In Figure 5, the magnetic susceptibilities for the
AuBa2Ca2Cu3O9.0 and
AuBa2Ca3Cu4O11.0 samples are shown.
The Au-1234-Ba phase undergoes the superconducting transition at
Tc = 99 K and has large enough diamagnetism at 5 K. On the
other hand, in the as-grown Au-1223-Ba sample, the decrease of the magnetic
susceptibility starts at  90 K but both Meissner
and shielding fractions are very small even at 5 K.
90 K but both Meissner
and shielding fractions are very small even at 5 K.
To change the hole concentration of Au-1223-Ba, the sample was postannealed
for 2 h at 300 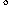 C under oxygen atmosphere.
According to the thermogravimetric analysis, the weight decrease by the
postannealing is
C under oxygen atmosphere.
According to the thermogravimetric analysis, the weight decrease by the
postannealing is  0.13%, which corresponds to
removal of
0.13%, which corresponds to
removal of  0.07 oxygen atoms or a decrease of
0.14 holes per unit formula. After the postannealing, the superconducting volume
fraction increased noticeably, as shown in Figure 5. However, the
superconducting transition is still very broad. Weak diamagnetism is seen below
0.07 oxygen atoms or a decrease of
0.14 holes per unit formula. After the postannealing, the superconducting volume
fraction increased noticeably, as shown in Figure 5. However, the
superconducting transition is still very broad. Weak diamagnetism is seen below
 90 K in the postannealed sample, but the main
transition occurs around 30 K, which appears to be the Tc of
Au-1223-Ba. The
90 K in the postannealed sample, but the main
transition occurs around 30 K, which appears to be the Tc of
Au-1223-Ba. The  90 K transition is likely due
to the Au-1234-Ba phase, although undetected by XRD. These results may suggest
that the as-grown Au-1223-Ba phase lies in the overdoped state of holes.
90 K transition is likely due
to the Au-1234-Ba phase, although undetected by XRD. These results may suggest
that the as-grown Au-1223-Ba phase lies in the overdoped state of holes.
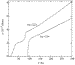
The electric resistivities of Au-1223-Ba and Au-1234-Ba are given in Figure
6. The normal-state temperature dependencies reveal the metallic nature for both
phases. In Au-1234-Ba, the onset temperature is 108 K and zero-resistivity is
attained at 93 K. As expected from the magnetic data, two-step superconducting
transitions were observed for the as-grown Au-1223-Ba sample at 86 K and  40 K with a zero-resistivity temperature of 13 K.
Both phases indicate a large residual resistivity with extrapolation of the
normal-state resistivity to zero temperature. Impurity phases contained in both
samples could be a reason for the large residual resistivity. We need to obtain
a single-phase sample to elucidate this problem, and such an attempt is in
progress.
40 K with a zero-resistivity temperature of 13 K.
Both phases indicate a large residual resistivity with extrapolation of the
normal-state resistivity to zero temperature. Impurity phases contained in both
samples could be a reason for the large residual resistivity. We need to obtain
a single-phase sample to elucidate this problem, and such an attempt is in
progress.
Conclusions
Two members of a new Au-based
AuBa2Can-1CunO2n+3
family with n = 3, 4 (Au-1223-Ba and Au-1234-Ba) were synthesized by
high-pressure/high-temperature conditions at 6 GPa and 1250-1300 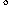 C. The X-ray diffraction and high-resolution
transmission electron microscopy measurements show a M-12(n-1)n-type structure
with
BaO-AuO-BaO-CuO2-(Ca-CuO2)n-1
stacking of planes along the c-axis. The Au-1234-Ba phase showed bulk
superconductivity below Tc = 99 K. The superconducting
transition of Au-1223-Ba is very broad with a small volume fraction even at 5 K.
Some oxygen was lost from the Au-1223-Ba phase after postannealing at 300
C. The X-ray diffraction and high-resolution
transmission electron microscopy measurements show a M-12(n-1)n-type structure
with
BaO-AuO-BaO-CuO2-(Ca-CuO2)n-1
stacking of planes along the c-axis. The Au-1234-Ba phase showed bulk
superconductivity below Tc = 99 K. The superconducting
transition of Au-1223-Ba is very broad with a small volume fraction even at 5 K.
Some oxygen was lost from the Au-1223-Ba phase after postannealing at 300 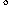 C in O2, which leads to a noticeable
increase of the superconducting volume fraction. This result suggests an
overdoped state of carriers in the as-grown Au-1223-Ba
C in O2, which leads to a noticeable
increase of the superconducting volume fraction. This result suggests an
overdoped state of carriers in the as-grown Au-1223-Ba
Acknowledgment
The authors would like to thank M. Akaishi and S. Yamaoka for helpful suggestions on high-pressure synthesis, K. Kosuda for the EPMA measurements, and S. Takenouchi for the carbon analysis. This work was supported by the Multi-Core Project and Special Coordination Funds of the Science and Technology Agency of the Japanese Government.
* Corresponding author.
 STA fellow of NIRIM. On leave from
Inorganic Chemistry Department, Moscow State University, Moscow, Vorobyevy Gory
119899, Russia.
STA fellow of NIRIM. On leave from
Inorganic Chemistry Department, Moscow State University, Moscow, Vorobyevy Gory
119899, Russia.
.gif) Present address:
Department of Chemistry, Princeton University, Princeton, NJ
08544.
Present address:
Department of Chemistry, Princeton University, Princeton, NJ
08544.
1. Wells, A. F. Structural Inorganic Chemistry, 4th ed.; Clarendon Press: Oxford, 1975.
2. Shannon, R. D. Acta Crystallogr. A 1976, 32,
751.
3. Bordet, P.; Le Floch, S.; Chaillout, C.; Duc, F.; Gorius, M. F.; Perroux,
M.; Capponi, J. J.; Toulemonde, P.; Tholence, J. L. Physica C
1997, 276, 237.
4. Bordet, P.; Le Floch, S.; Capponi, J. J.; Chaillout, C.; Gorius, M. F.;
Marezio, M.; Tholence, J. L.; Radaelli, P. G. Physica C 1996,
262, 151.
5. Takayama-Muromachi, E. Chem. Mater. 1998, 10,
2686.
6. Kawashima, T.; Matsui, Y.; Takayama-Muromachi, E. Physica C
1994, 224, 69.
7. Sheng, Z. Z.; Hermann, A. M. Nature 1988, 332,
55.
8. Takayama-Muromachi, E.; Matsui, Y.; Kosuda, K. Physica C
1995, 241, 137.
9. Kawashima, T.; Matsui, Y.; Takayama-Muromachi, E. Physica C
1995, 254, 131.
10. Isobe, M.; Kawashima, T.; Kosuda, K.; Matsui, Y.; Takayama-Muromachi, E.
Physica C 1994, 234, 120.
11. Vaughey, J. T.; Thiel, J. P.; Hasty, E. F.; Groenke, D. A.; Shtern, C.
L.; Poeppelmeier, K. R.; Dabrowski, B.; Hinks, D. G.; Mitchell, A. W. Chem.
Mater. 1991, 3, 935.
12. Dabrowski, B.; Radaelli, P.; Hinks, D. G.; Mitchell, A. W.; Vaughey, J.
T.; Groenke, D. A.; Poeppelmeier, K. R. Physica C 1992,
193, 63.
13. Takayama-Muromachi, E.; Isobe, M. Jpn. J. Appl. Phys. 1994,
33, L1399.