



As recently as 2010 it wasn't known if room temperature superconductivity was even possible. Today Superconductors.ORG announces the discovery of two new superconductors with transition temperatures (Tc) more than 100 degrees above room-temperature. Sn9SbTe3Ba2MnCu14O28+ and Sn8SbTe4Ba2MnCu14O28+ are the 23rd and 24th compounds found to superconduct above room temperature, with the latter being the first to go above 400 Kelvin.
Confirmation of superconductivity in these two materials was made through numerous resistance and magnetization tests. The average Tc of eight resistance tests of Sn8SbTe4 was 128.8 Celsius. Eight magnetization tests of the same material then produced an average Tc of 129.4 Celsius. The two plots at page top show both heating and cooling magnetization tests with a diamagnetic (Meissner) transition where the straight lines skew apart. Those lines approximate the average of the data points. The plot jitter results from a high thermal noise level and low volume fraction of the superconductive phase.

|
Below are plots of companion resistance tests of Sn8SbTe4. Both heating and cooling are shown. The bulk (non-superconductive host) material has a negative temperature coefficient, sloping the opposite direction as the superconductive transitions. These plots confirm a new world record for high Tc. 
 |
400K superconductivity was achieved by taking the 119C formulation, announced in February 2015, and adding one atom each of antimony and copper to alter the target structure from N212 to P212 (shown above left). This created the compound Sn9SbTe3Ba2MnCu14O28+ which produced a Tc near 124 Celsius. The primary purpose of adding antimony was to increase Tc and harden the pellet. But it also gave the (non-superconducting) bulk a measurable resistance, which the 119C material did not have. Resistance in the bulk is necessary in order to observe the resistive transition at Tc. The Sn9SbTe3 plots are shown below.


The next step getting to 400K was to increase the tellurium content by one atom, while reducing the tin content by the same amount. This increased the overall dielectric constant (K) in the insulating layers. Though this action over-dopes the insulating layers with electrons, the increased Kappa promotes Tc an additional 5 degrees to near 129C. The resulting formula then becomes Sn8SbTe4Ba2MnCu14O28+.
The dots within the below grid show how Tc has advanced in the copper-oxides through correspondingly higher planar weight ratios. Those compounds in the yellow box not only have the highest planar weight ratios, they feature a high dielectric constant (K) as well. At the same PWR, the high Kappa compounds have significantly higher transition temperatures.

In order to maximize formation of the P212 structure, Sn8SbTe4Ba2MnCu14O28 was synthesized using the layer cake method, as shown below. The pellet had approximately 100 interference layers. And, even using this layering technique, the volume fraction is low, requiring very sensitive test equipment.
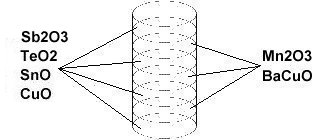
Stoichiometric ratios of the below chemicals were used for the ODD layers:
SnO 99.9% (Alfa Aesar) 7.80 grains...and the below ratios for the EVEN layers.
BaCuO 99.9% (Alfa Aesar) 3.14 grainsThe chemical precursors were pelletized at 60,000 PSI and pre-sintered for 24 hours at 715C. The pellet was then sintered for 10 hours at 880C and annealed for 10+ hours at 500C in flowing O2. Temperature was determined using an Omega type "T" thermocouple and precision OP77 DC amplifier. The magnetometer employed twin Honeywell SS94A1F Hall-effect sensors with a tandem sensitivity of 50 mv/Gauss.
RESEARCH NOTE: The copper-oxides are strongly hygroscopic. All tests should be performed immediately after annealing.
RE-PUBLICATION NOTICE: Elsevier Publishing, dba Elsevier Science, as well as Morris Communications, both print and broadcast divisions, are specifically prohibited from re-publishing any part of this news story.
E. Joe Eck
© 2015 Superconductors.ORG
All rights reserved.
1. Testing temperatures are believed accurate within ± 0.5 degrees C.
2. Dielectric constants were obtained from H. P. R. Frederikse, CRC Handbook of Chemistry and Physics.
 BACK to "News" page at Superconductors.ORG
BACK to "News" page at Superconductors.ORG