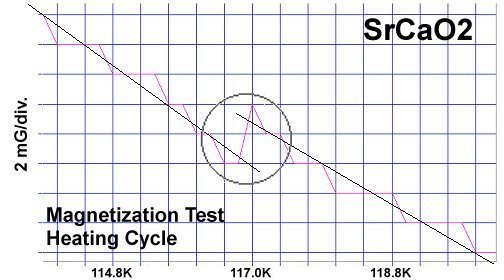
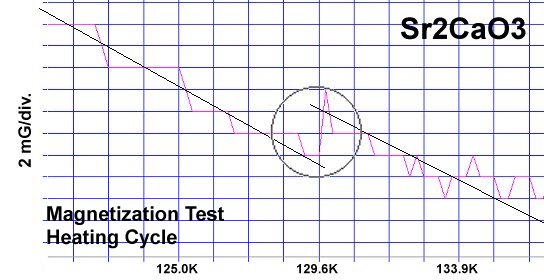


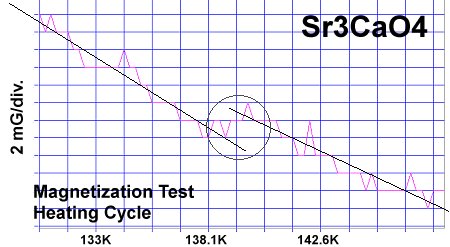
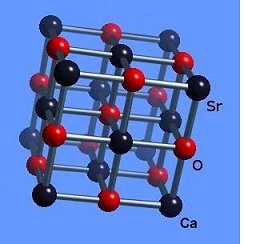
The strontium-calcium-oxygen compounds SrCaO2, Sr2CaO3 and Sr3CaO4 have all been found to produce Meissner transitions above 110 Kelvin. Alkali-earths were chosen because of their +2,+1 oxidation states and because they have collinear layered structures as oxides. (See the cubic structure of the prototype SrCaO2 at lower left.) Magnetization plots of SrCaO2 and Sr2CaO3 are shown at page top. Sharp diamagnetic (Meissner) transitions appear near 117 Kelvin and 129K respectively. Both transitions are around 4-5 milligauss. Above is a magnetization plot of Sr3CaO4, which produced the highest Tc of the three near 139K. The volume fraction of all three of these discoveries was low.
|
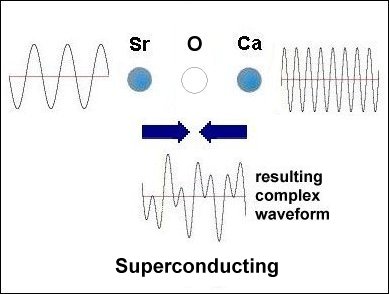
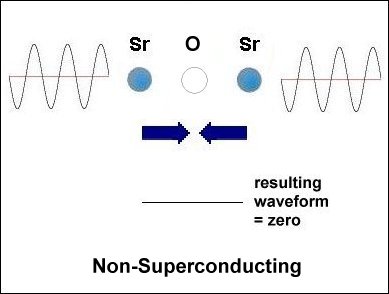
Having only 3 elements and a layered structure, the mechanism that makes superconductivity possible must be a simple one. It is already known that weight disparity must exist on opposite sides of the oxygen atoms. All known high-temperature superconductors can be shown to have it. Additionally, weight disparity is the only way to achieve periodic compression. Since the adjacent metals that facilitate superconductivity all have +2,+1 oxidation states, those states must be shifting back and forth in sync with the compression rate. This "virtual" redox does not involve the gain or loss of any elements. So very little energy is required to shift the ionic charge.[1] The below graphics illustrate the process by which superconductivity is achieved in this ternary system. In the below left box the sinusoidal waveforms near the Sr and Ca atoms show the relative frequency of each atom's vibration along the C axis. Since strontium is heavier than calcium, it oscillates at a lower frequency, producing fewer cycles per unit time. The result is that different vibrational frequencies heterodyne at the oxygen layer, producing periodic compression proportional to a complex waveform. However, in the SrO (non-superconducting) structure to the right, the waveforms cancel at the oxygen layer, due to identical frequencies producing a null heterodyne. Thus, a difference in atomic mass is essential to achieving periodic compression at the oxygen site.[2] |
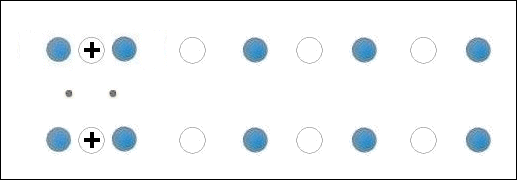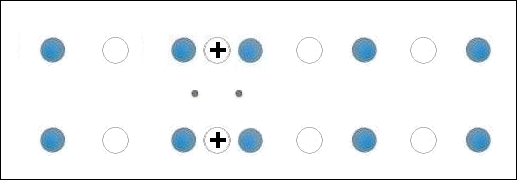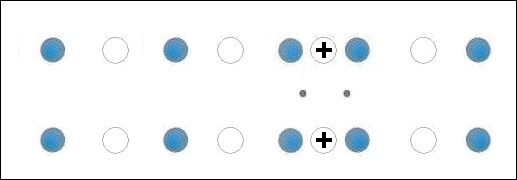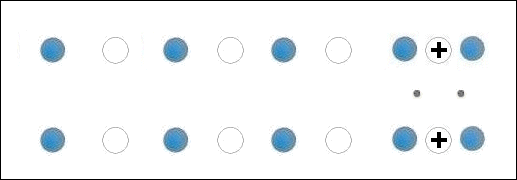
In the below animation periodic compression creates a moving positive charge that briefly appears at each oxygen site. This is because the oxygen valency is no longer satisfied when the adjacent metals change state from +2 to +1. The red dots between the positively-charged oxygen ions are paired electrons. This process is similar to the phonon-mediated coupling of Type 1 superconductors.
The chemical precursors were pelletized at 60,000 PSI and sintered for 10 hours at 880C. They were then annealed for 10+ hours at 500C in flowing O2. Temperature was determined using an Omega type "T" thermocouple and precision OP77 DC amplifier. The magnetometer employed twin Honeywell SS94A1F Hall-effect sensors with a tandem sensitivity of 50 mv/Gauss.
RESEARCH NOTES: The oxides are both brittle and strongly hygroscopic. All tests should be performed immediately after annealing.
RE-PUBLICATION NOTICE: Elsevier Publishing, dba Elsevier Science, as well as Morris Communications, both print and broadcast divisions, are specifically prohibited from re-publishing any part of this news story.
E. Joe Eck
© 2016 Superconductors.ORG
All rights reserved.
 BACK to "News" page at Superconductors.ORG
BACK to "News" page at Superconductors.ORG