
The Type 1 category of superconductors is mainly comprised of metals and metalloids that show some conductivity at room temperature. They require incredible cold to slow down molecular vibrations sufficiently to facilitate unimpeded electron flow in accordance with what is known as BCS theory. BCS theory suggests that electrons team up in "Cooper pairs" in order to help each other overcome molecular obstacles - much like race cars on a track drafting each other in order to go faster. Scientists call this process phonon-mediated coupling because of the sound packets generated by the flexing of the crystal lattice.
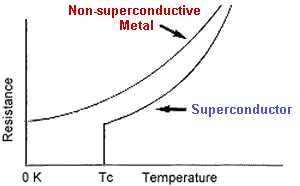
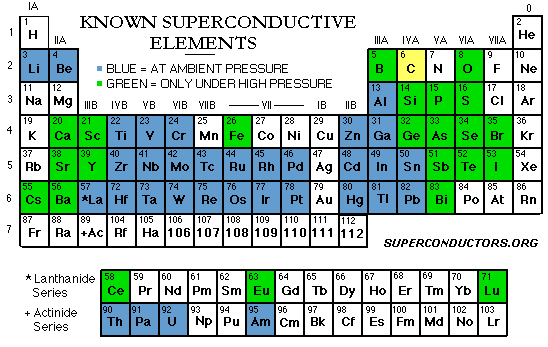
Type 1 superconductors - characterized as the "soft" superconductors - were discovered first and require the coldest temperatures to become superconductive. They exhibit a very sharp transition to a superconducting state (see above graph) and "perfect" diamagnetism - the ability to repel a magnetic field completely. Below is a list of known Type 1 superconductors along with the critical transition temperature (known as Tc) below which each superconducts. The 3rd column gives the lattice structure of the solid that produced the noted Tc. Surprisingly, copper, silver and gold, three of the best metallic conductors, do not rank among the superconductive elements. Why is this ?
![]()
|
Lead (Pb) Lanthanum (La) Tantalum (Ta) Mercury (Hg) Tin (Sn) Indium (In) Palladium (Pd)* Chromium (Cr)* Thallium (Tl) Rhenium (Re) Protactinium (Pa) Thorium (Th) Aluminum (Al) Gallium (Ga) Molybdenum (Mo) Zinc (Zn) Osmium (Os) Zirconium (Zr) Americium (Am) Cadmium (Cd) Ruthenium (Ru) Titanium (Ti) Uranium (U) Hafnium (Hf) Iridium (Ir) Beryllium (Be) Tungsten (W) Platinum (Pt)* Lithium (Li) Rhodium (Rh) |
7.196 K 4.88 K 4.47 K 4.15 K 3.72 K 3.41 K 3.3 K 3 K 2.38 K 1.697 K 1.40 K 1.38 K 1.175 K 1.083 K 0.915 K 0.85 K 0.66 K 0.61 K 0.60 K 0.517 K 0.49 K 0.40 K 0.20 K 0.128 K 0.1125 K 0.023 K (SRM 768) 0.0154 K 0.0019 K 0.0004 K 0.000325 K |
FCC HEX BCC RHL TET TET (see note 1) (see note 1) HEX HEX TET FCC FCC ORC BCC HEX HEX HEX HEX HEX HEX HEX ORC HEX FCC HEX BCC (see note 1) BCC FCC |
*Note 1: Tc's given are for bulk (alpha form), except for Palladium, which
has been irradiated with
He+ ions, Chromium as a thin film,
and Platinum as a compacted powder.
![]()
**Note 2: Normally bulk carbon (amorphous, diamond, graphite, white) will not superconduct at any temperature. However, a Tc of 15K has been reported for elemental carbon when the atoms are configured as highly-aligned, single-walled nanotubes. And non-aligned, multi-walled nanotubes have shown superconductivity near 12K. Since the penetration depth is much larger than the coherence length, nanotubes would be characterized as "Type 2" superconductors.
***Note 3: For a list of elements that are naturally diamagnetic, click HERE.
![]()
Author's Comment: The information posted on this page was obtained from
a variety of sources including, but not limited to, the CRC Handbook of Chemistry
and Physics, the Technische Universität München, Reade Metals and Minerals Corp., industry news sources,
and various private researchers.
A special thanks to Professor Bertil Sundqvist, Department of Experimental Physics,
Umea University, Sweden, also to Dr. Jeffery Tallon, Industrial Research Ltd., New Zealand,
and to Dr. James S. Schilling, Department of Physics, Washington University.
[Last page rev: March 2023]
![]()