Superconductor Terminology
and the Naming Scheme
![]()

![]()
Anneal: To heat and then slowly cool a material to reduce brittleness. Annealing of ceramic superconductors usually follows sintering and is done in an oxygen-rich atmosphere to restore oxygen lost during sintering. The oxygen content of a ceramic superconductor is critical. For example, YBCO with 6.4 atoms of oxygen will not superconduct. But YBCO with 6.5 atoms will. Click here to see a graphical representation of this.
Anti-ferromagnetism: A state of matter
where adjacent ions in a material are aligned in opposite or "anti-parallel"
arrays. Such materials display almost no response to an external magnetic
field at low temperatures and only a weak attaction at higher temperatures.
There is evidence that anti-ferromagnetism in the copper oxides plays
a role in the formation of Cooper pairs and, thus, in facilitating a superconductive
state in some compounds.
BCS Theory: The first widely-accepted
theory to explain superconductivity put forth in 1957 by John Bardeen,
Leon Cooper, and John Schreiffer. The theory asserts that, as electrons
pass through a crystal lattice, the lattice deforms inward towards the
electrons generating sound packets known as "phonons". These phonons produce
a trough of positive charge in the area of deformation that assists subsequent
electrons in passing through the same region in a process known as phonon-mediated coupling.
This is analogous to rolling
a bowling ball up the middle of a bed. 2 people, one lying on each side
of the bed, will tend to roll toward the center of the bed, once the ball
has created a depression in the mattress. And, a 2nd bowling ball, placed
at the foot of the bed, will now, quite easily, roll toward the middle.
For a more technical explanation click here.
Borocarbides: Superconducting borocarbides are compounds containing both boron and carbon in combination with rare-earth and transition elements; some of which exhibit the unusual ability to return to a normal, non-superconductive state at temperatures below Tc. For more on this, click here.
BSCCO: An acronym for a ceramic superconductor system containing the elements Bismuth, Strontium, Calcium, Copper and Oxygen. Typically, a small amount of lead is also included in these compounds to promote the highest possible Tc. BSCCO has probably found the widest acceptance among high-Tc superconductor applications due to its unique properties. BSCCO compounds exhibit both an intrinsic Josephson effect and anisotropic (directional) behavior. You can view a list of BSCCO compounds on the "Type 2" page.
Ceramics: Ceramic superconductors are inorganic compounds formed by reacting a metal with oxygen, nitrogen, carbon or silicon. The best-known of these are the copper-perovskites. Ceramics are typically hard, brittle, heat-resistant materials formed by a process known as solid-state reaction.
Charge Reservoirs: In superconductors,
charge reservoirs are the layers that may control the oxidation
state of adjacent superconducting planes (even though they themselves are
not superconducting). In the layered cuprates, these consist of copper-oxide
chains.
Chevrel (phases): A class of molybdenum chalcogenides (compounds containing Group VI elements S, Se or Te along with molybdenum and a positively charged metal ion) - named for Roger Chevrel of the University of Rennes, whose research brought them to the attention of the scientific community in the early 1970's. Recently, the Superconductivity Group at the University of Durham (UK) reported a novel fabrication technique that increases Hc2 (upper critical field) in the chevrel PbMo6S8 from 50 T in bulk materials up to > 100 T. Click HERE to read a [technical] writeup on this (as a PDF file). Or click HERE to see a short list of some of these compounds alongside their Tc's.
Coherence Length: The size of a cooper pair - representing the shortest distance over which superconductivity can be established in a material. This is typically on the order of 1000Å; although it can be as small as 30Å in the copper oxides.
Cooper Pair: Two electrons that appear to "team up" in accordance with theory - BCS or other - despite the fact that they both have a negative charge and normally repel each other. (Named for Leon Cooper.) Below the superconducting transition temperature, paired electrons form a condensate - a macroscopically occupied single quantum state - which flows without resistance. However, since only a small fraction of the electrons are paired, the bulk does not qualify as being a "bose-einstein condensate". Click here to see an animation of a cooper pair.
DAC: An acronym for "diamond anvil cell". Often the Tc of a superconductor can be coaxed upward with the application of high pressure. The DAC is used to accomplish this in the laboratory. A DAC is composed of 2 specially-cut diamonds and a stainless steel gasket. The gasket goes between the diamonds and seals a small chamber in which a fluid is placed. Since neither the diamonds nor the liquid will compress, hydrostatic forces in excess of a million atmospheres can be brought to bear on a sample suspended within the fluid. Click here to see a graphic of a DAC.
Diamagnetism: The ability of a material to repel a magnetic field. Many naturally-occurring substances (like water, wood and paraffin, and many of the elements) exhibit weak diamagnetism. Superconductors exhibit strong diamagnetism below Tc. In a few rare compounds, a material may become superconductive at a higher temperature than the point at which diamagnetism appears. But, as a rule, the onset of strong diamagnetism is one of the most reliable ways to ascertain when a material has become superconductive. To see a short movie of a magnet being levitated by a superconductor, click here.
D-Wave: A form of electron pairing in which the electrons travel together in orbits resembling a four-leaf clover. Wave functions help theoreticians describe (and predict) electron behavior. The d-wave models have gained substantial support recently over s-wave pairing as the mechanism by which high-temperature superconductivity might be explained. Click here to see a graphic.
Energy Gap: This is the energy required to break up a pair of electrons. According to BCS theory, the formula for determining the energy gap (in meV) is Eg=7/2 KTc. Where K = Boltzmann's constant (8.62e-5 eV/K). And where Tc is the critical transition temperature in Kelvin. Since electron-pairing is universally agreed to be the method by which superconductivity occurs, this is the amount of energy required to disrupt the superconducting state.
ESR: An acronym for "Electron Spin Resonance" (also EPR: Electron Paramagnetic Resonance). This is another mechanism by which superconductivity might be explained in some materials. Simply put, ESR is the response of electrons to electromagnetic radiation or magnetic fields at discrete frequencies. Electrons, as they move, create tiny magnetic moments. Nearby electrons are influenced either beneficially or adversely. When the moments are complementary, the electrons become paired and can help each other move through a crystal lattice.
Ferrite: Ferrites are ceramics with magnetic properties. They are included on this page because many of the same elements used in ferrites (e.g. Ba, Sr, Tm, O) are also key constituents in ceramic superconductors. This may be an important clue in understanding high-temperature superconductivity.
Ferromagnetism: A state wherein a material exhibits magnetization through the alignment of internal ions (neighboring magnetic moments). This contrasts with paramagnetism, which is temporary, much weaker and results from unpaired electrons.
Flux-Lattice: A configuration created when flux lines from a strong magnetic field try to penetrate the surface of a Type 2 superconductor. The tiny magnetic moments within each resulting vortex repel each other and a periodic lattice results as they array themselves in an orderly fashion.
Fluxon: The smallest magnetic flux (flux quantum) that exists in nature. Just as electrons are quantized charge, fluxons are a quantized flux. The term is used in association with vortices, which result from magnetic fields penetrating Type 2 superconductors in single fluxon quanta. Click here to view a hollographic depiction of waves of fluxons on the surface of superconducting Niobium.
Flux-Pinning: The phenomenon where a magnet's lines of force (called flux) become trapped or "pinned" inside a superconducting material. This pinning binds the superconductor to the magnet at a fixed distance. Flux-pinning is only possible when there are defects in the crystalline structure of the superconductor (usually resulting from grain boundaries or impurities). Flux-pinning is desirable in high-temperature ceramic superconductors in order to prevent "flux-creep", which can create a pseudo-resistance and depress Jc and Hc. Click here to see a superconductor suspended in air by flux-pinning.
Four-point Probe: The most common method of determining the Tc of a superconductor. Wires are attached to a material at four points with a conductive adhesive. Through two of these points a voltage is applied and, if the material is conductive, a current will flow. Then, if any resistance exists in the material, a voltage will appear across the other two points in accordance with Ohm's law (voltage equals current times resistance). When the material enters a superconductive state, its resistance drops to zero and no voltage appears across the second set of points. By using the four-point method, instead of just two points, resistance in the adhesive and wires can be ignored; as the second set of points do not themselves conduct any current and can, therefore, only reflect what voltage exists across the body of the material.
Hall Effect: When a magnetic field is applied perpendicularly to a thin metal film or semiconductor film that is conducting an electric current, a small voltage will appear perpendicular to the axis of both the film and the magnetic field. This voltage is proportional to the strength of the applied field. However, the output is typically not linear. The Hall resistance (the ratio of the Hall voltage to the current) changes in steps, pursuant to the laws of quantum mechanics. This is known as the Integral Quantum Hall Effect or just Quantum Hall Effect. Discovered in 1879, the Hall effect was named for its discoverer Edwin H. Hall, a graduate student at Johns Hopkins University.
Hc: The scientific notation representing the "critical field" or maximum magnetic field that a superconductor can endure before it is "quenched" and returns to a non-superconducting state. Usually a higher Tc also brings a higher Hc.
Heavy Fermions: Compounds containing the elements cerium, ytterbium or uranium; whose (inner shell) conduction electrons often have effective masses (called quasiparticle masses) several hundred times as great as that of a "free" (normal) electron mass. This gives them what's known as a low "Fermi energy" and makes them unlikely - and unusual - superconductors. Research suggests cooper-pairing in heavy fermion systems arises from the magnetic interactions of the electron spins.
Hole: A positively-charged vacancy within a crystal lattice resulting from the shortage of an electron in that region. Holes are typically induced by doping a material with an impurity. However, they can also be synthesized electronically with devices like the field-effect transistor (FET). Modern electronic devices rely heavily on holes (as p-type semiconductors) to function. There is evidence that the holes of hypocharged oxygen in charge-reservoirs are, in fact, what makes possible high-temperature superconductivity in the layered cuprates.
HTS: An acronym for "High-Temperature Superconductor" (or Superconductivity). There is no widely-accepted temperature that separates HTS from LTS (Low-Temperature Superconductors). However, all the superconductors known before the 1986 discovery of the superconducting oxocuprates would be classified LTS. The barium-lanthanum-cuprate fabricated by Müller and Bednorz, with a Tc of 30K, is generally considered to be the first HTS material. Certainly any compound that will superconduct above the boiling point of liquid nitrogen at 77 Kelvin would be HTS.
Hysteresis (loop): Hysteresis, as it applies to a superconductor, relates to the dynamic response of a superconductor to a strong magnetic field impinged upon it. As the strength of a nearby magnetic field (H) increases, the critical transition temperature (Tc) of a superconductor will decrease. And, at some point superconductivity will completely disappear, as it becomes "quenched". However, as the magnetic field is gradually withdrawn, the superconductor may NOT immediately return to a superconductive state. Herein lies the hysteresis. The graph of H-vs-Tc is different retreating than it is advancing (creating a "loop" shape). This fact must be weighed carefully in high-current applications where the superconductor Hc may - even briefly - be exceeded, as significant power losses can result.
Infinite layer: Infinite layer compounds have no clear separation between molecules. Rather than electrostatic bonding between discrete molecules to form a bulk crystalline aggregate, all the atoms are bound together by covalent or co-ionic bonding to form the equivalent of one huge molecule. (Ba,Sr)CuO2 and Na2Ba6Si46 are examples of "infinite layer" or "infinite network" superconductor compounds.
Isotope Effect: The influence atomic mass contributes to the critical transition temperature of a superconductor. For example, 203.4Hg has a Tc of 4.126K. While 198Hg has a Tc of 4.177K. Since both forms of mercury have the same lattice structure, this difference in Tc can be attributed solely to the difference in mass. To learn more, click here.
Jc: The scientific notation representing the "critical current density" or maximum current that a superconductor can carry. Also note that, as the current flowing through a superconductor increases, the Tc will usually decrease.
Josephson Effect (also DC Josephson Effect): A phenomenon named for Cambridge graduate student Brian Josephson, who predicted that electrons would "tunnel" through a narrow (<10 angstroms) non-superconducting region, even in the absence of an external voltage. In a normal conductor, electrical current only flows when there's a voltage differential and contiguous electrical connection. It has been theorized that the Josephson Effect arises from the incoherent phase relationships between superconducting electrons in the two (separated) superconductors. The AC Josephson Effect is where the current flow oscillates as an external magnetic field impinged upon it increases beyond a critical value. [at a frequency of 2eV/h, where e is the electron charge, V is the voltage that appears, and h is Planck's constant] Sidebar: This oscillation frequency has, in fact, resulted in an upward revision of Planck's constant from 6.62559e-34 to 6.626196e-34.
Josephson Junction: A thin layer of insulating material sandwiched between 2 superconducting layers. Electrons "tunnel" through this non-superconducting region in what is known as the "Josephson effect" (see above). Sidebar: The standard volt is now defined as the voltage required to produce a frequency of 483,597.9 GHz in a Josephson Junction.
Kelvin: A scale of temperature measurement that starts at "absolute zero", the coldest theoretical temperature attainable. (Named for Lord William Thomson Kelvin.)
Meissner Effect: Exhibiting diamagnetic properties to the total exclusion of all magnetic fields. (Named for Walther Meissner.) This is a classic hallmark of superconductivity and can actually be used to levitate a strong rare-earth magnet. To see a movie of a magnet being levitated by a superconductor, click here.
Mott Transition: The Mott transition is the shift from an insulating to a metallic state in a material. The high-temperature copper oxides are composed of CuO2 planes that are separated from each other by ionic "blocking layers". Although it has one conduction electron (or hole) per Cu site, each CuO2 plane is originally insulating because of the large electron correlation. That behavior is typical of the Mott insulator state, in which all the conduction electrons are tied to the atomic sites. The superconducting state emerges when holes from the blocking layers dope the CuO2 layers in a way that alters the number of conduction electrons and triggers the Mott transition. Researchers believe that the strong antiferromagnetic correlation, which originates in the Mott-insulating CuO2 sheets and persists into the metallic state, could be a possible mechanism of high-temperature superconductivity. (courtesy Science Week) Mott "transients" have also been observed in some materials that have a low-volume-fraction superconductor embedded in a semi-conductive bulk. To read more about this, click HERE.
Organics: Organic superconductors are a sub-class of organic conductors that include molecular salts, polymers and pure carbon systems (including carbon nanotubes and C60 compounds). They may also be referred to as "molecular" superconductors. They are typically large, carbon-based molecules of 20 or more atoms, consisting of a planar organic molecule and a non-organic anion. For a non-technical write-up on organics, click here. Or, for a more technical paper on this subject, click here.
Penetration Depth (also London Penetration Depth): This term relates to how deeply a magnetic field will penetrate the surface of a superconductor. An external magnetic field impinged upon a Type 2 superconductor will decay exponentially into the surface based on the paired electron density within the superconductor (only a small fraction of the electrons are in a superconductive state). The "London" name comes from brothers Fritz London and Heinz London, who in 1935 created a theoretical model of superconductivity. For a more technical explanation and the actual formula to calculate penetration depth, click here.
Perovskites: A large family of crystalline ceramics that derive their name from a mineral known as a perovskite. They are the most abundant minerals on earth and have a metal-to-oxygen ratio of approximately 2-to-3. Copper-oxide superconductors are layered perovskites. The perovskite name comes from Russian mineralogist Count Lev Aleksevich von Perovski.
Phase-Slip (also Quantum Phase-Slip): A point where a material in a superconductive state spontaneously changes from one state to another, generating a topological "defect". This defect causes paired electrons to become "out of step" with each other, producing a voltage and, ergo, non-zero electrical resistance. This phenomenon has been observed in ultra-thin wires less than a few tens-of-nanometers in diameter. Though bulk superconductivity may persist (T<Tc), one consequence of phase slip is a lower current-carrying state. A similar phenomenon occurs in Josephson Junctions.
Planar Weight Disparity (PWD): A term referring to the method by which Tc can often be increased by adjusting the relative weights of alternating layers in copper-oxide superconductors. The greatest improvements usually occur when PWD is applied along both the C1 and C2 axes simultaneously. This weight disparity results in periodic compression of interposed oxygen atoms and may be an essential component of high temperature superconductivity. Click HERE to read the original news story announcing this discovery in 2005.
Proximity Effect: The phenomenon where a thin film of non-superconductive material in close proximity with a superconductor takes on superconductive properties. The Josephson junction is a device that takes advantage of this phenomenon. The Inverse Proximity Effect is where just the opposite occurs. A non-superconductive metal can enhance the Tc of an adjacent superconductor. This inverse effect has been observed with silver and lead.
P-Wave: A rare form of electron pairing in which two electrons travel together in spherical orbits; with both having the same direction of rotation. (See "D-Wave" explanation above.)
Quasiparticle: A bare particle that is "dressed" or "clothed" by a cloud of other surrounding particles. Quasiparticles behave similarly to bare (normal) particles, but usually have a larger effective mass due to this cloud moderating interactions with other particles.
Quench: The phenomenon where superconductivity in a material is suppressed; usually by exceeding the maximum current the material can conduct (Jc) or the maximum magnetic field it can withstand (Hc).
Re-entrant (behavior): A condition where a material retreats from its superconductive state and then re-enters it. This can be caused by a strong external magnetic field that dynamically exceeds the Hc of the material and/or is mis-aligned (in the case of some organic superconductors), a discordant temperature below Tc (in the case of some borocarbides), or by Jc hysteresis (momentarily exceeding the critical current density, causing the Tc to shift downward).
Resistance: The opposition of a material to the flow of electrical current through it. Energy lost due to resistance is a result of vibrations at the molecular level and manifests itself as heat in proportion to the square of the current flow. In a superconductor all resistance disappears below a certain temperature. However, this applies only to direct current (DC) electricity. Other types of losses result when transporting alternating current (AC). Examples of this include hysteresis, reactive-coupling and radiational losses. In the new high-temperature ceramic superconductors, the power loss in applications like transmission lines is inversely proportional to the critical current density for low magnetic field applications. This limitation can be compensated for to some degree by increasing the ratio of voltage to current. In Type 2 superconductors carrying high-frequency alternating current, "skin effect" losses also result as the energy tends to migrate to the surface where the conductive medium is incontiguous, producing a pseudo-resistance. In some materials the amount of resistance may also depend on the direction of current flow (anisotropic resistivity) and/or presence of an external magnetic field (hall effect).
Room-temperature Superconductivity (RTSC): It has been theorized that a metallic form of hydrogen might be a room-temperature superconductor. In 1996 physicists at Lawrence Livermore Laboratory were able to briefly create metallic hydrogen. But, its existence was fleeting and no measurement of the Meissner effect was possible. Zero resistance has been observed at room temperatures in ballistic quantum wire. However, having one-dimensional geometry, this wire does not exhibit the Meissner effect, except when configured as a closed loop. Since 2011 many oxide superconductors have been discovered with signs of RTSC. But the volume fraction (percentage) of material was so low as to be unusable until a refinement method can be developed. To read more on the latest of these discoveries, click HERE.
Sinter: The process of heating a material to just below its melting point. An extended period of sintering is the method by which the constituent components of a ceramic superconductor are combined in a solid-state reaction. Since ceramic superconductors are inherently brittle, sintering helps promote intergranular bonding and hardness.
SQUID: A superconducting loop interrupted in 2 places by Josephson junctions. When sufficient electrical current is conducted across the squid body, a voltage is generated proportional to the strength of any nearby magnetic field. The SQUID, an acronym for Superconducting QUantum Interference Device, is the most sensitive detector known to science. Click here to see a graphic.
Stripes: Stripes are microscopic rivers of charge that flow across the surface of a Type 2 superconductor. It is theorized that stripes encourage "holes" to pair up and, as such, may play a role in facilitating charge transfer. Recently, at the Stripes 2000 conference in Rome, Italy, it was shown that there exists a critical value of micro-strain that must be exerted upon the CuO2 planes for stripes to form. Click here to learn more.
Superconductor: An element, inter-metallic alloy, or compound that will conduct electricity without resistance below a certain temperature. However, this applies only to direct current (DC) electricity and to finite amounts of current. All known superconductors are solids. None are gases or liquids. Once set in motion, current will flow forever in a closed loop of superconducting material - making it the closest thing to perpetual motion in nature. Scientists refer to superconductivity as a "macroscopic quantum phenomenon". In addition to being classified Type 1 and Type 2, superconductors can be categorized further by their dimensionality. Most are 3-D. But some compounds, like surface-doped NaWO3 and some organic superconductors are 2-D. Li2CuO2 and single-walled carbon nano-tubes have shown rare 1-D superconductivity. In addition to repelling magnetic fields, enhanced thermal conductivity, higher optical reflectivity and reduced surface friction are also properties of superconductors. The term "superconductor" is also used in some instances to refer to materials that have near infinite thermal conductivity - such as carbon nanotubes. However, on this website it is used in the context of electrical conductivity only.
Susceptibility: A measure of the relative amount of induced magnetism in a material. Magnetic susceptibility is often used in lieu of resistance measurements to determine the transition temperature of a superconductor. Although, on occasion, the two techniques produce very different Tc's. In a typical superconductor, the (arbitrary) value of susceptibility will change from zero to a negative number as the temperature drops through Tc. However, in some materials it changes from positive to negative, as paramagnetism yields to diamagnetism.
S-Wave: A form of electron pairing in which the electrons travel together in spherical orbits, but in opposite directions. (See "D-Wave" explanation above.)
Tc: The scientific notation representing the critical transition temperature below which a material begins to superconduct. The sudden loss of resistance in a superconductive medium may occur across a range as small as 20 millionths of a degree or, in the case of some stoichiometrically imperfect compounds, tens of degrees. Click here to see a graphic example. ("Tc" is not to be confused with the atomic symbol for Technetium.)
Thin Film (Deposition): A method of fabricating ceramic superconductors to more precisely control the growth of the crystalline structure to eliminate grain boundaries and achieve a desired Tc. This can involve Pulsed-Laser Deposition (PLD) or Pulsed-Electron Deposition (PED) of the material. A variation of this technique can be used to increase the Tc of a superconductor by growing it on a supporting material with a smaller interatomic spacing. The supporting material acts as a molecular "girdle" to compress the atomic lattice of the superconductor, thereby raising its transition temperature. Superconductive tape is made using thin film deposition technology.
Translational Symmetry: As it applies to superconductivity, translational symmetry is where the process of charge transfer is repeated exactly as the charge carriers (paired electrons) traverse the solid. In a normal conductor, latent heat continuously vibrates the atomic lattice, deflecting mobile free electrons and preventing "perfect" translational symmetry. In a superconductor this scattering tendency is overcome.
Tungsten-bronze: A nebulous term used to describe alkali metal tungstenates, vanadates, molybdates, titanates and niobates. The term was originally coined to describe NaxWO3 compounds; the crystals of which look much like the copper-tin alloy known as bronze. There have been reports of superconductivity as high as 91K for a surface-doped sodium tungsten-bronze. This material was the first high-temperature superconductor discovered that does not contain any copper.
Ultraconductor: Materials known as ultraconductors™ display room-temperature resistance many orders of magnitude lower than the best metallic conductors. Examples of these materials include oxidized atactic polypropylene (OAPP) and some titanium-borides. Since ultraconductor™ is a colloquial term, these materials might better be described as "hyperconductors". The Meissner effect cannot be confirmed in them, but strong (giant) diamagnetism is in evidence. Some of them may actually find acceptance in high-current applications ahead of superconductors as a result of their low losses at ambient temperatures and pressures.
Undressing: The process by which a quasiparticle becomes more like a bare (normal) particle. It is theorized this may be a driving force behind superconductivity, as undressed electrons are significantly lighter and can, thus, conduct current more readily. To learn more, click here.
Unit Cell: A unit cell is the smallest assemblage of atoms, ions, or molecules in a solid, beyond which the structure repeats to form the 3-dimensional crystal lattice.
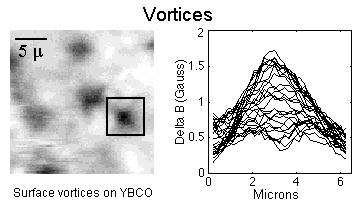
Vortices (plural of vortex): Swirling tubes of electrical current induced by an external magnetic field into the surface of a superconducting material that represent a topological singularity in the wavefunction. These are particularly evident in Type 2 superconductors during "mixed-state" behavior when the surface is just partially superconducting. Superconductivity is completely suppressed within these volcano-shaped structures. Recent research suggests that flux vortices may NOT possess quantum values (equal to multiples of Planck's constant divided by 2 times electron charge). But may instead have but a tiny fraction of the basic unit of magnetism. The movement of vortices can produce a pseudo-resistance and, as such, is undesirable. While superconductivity is a "macroscopic" phenomenon, vortices are a "mesoscopic" phenomenon. (See the graphic at the top of this page.)
YBCO: An acronym for a well-known ceramic superconductor composed of Yttrium, Barium, Copper and Oxygen. This was the first truly "high temperature" ceramic superconductor discovered; having a transition temperature well above the boiling point of liquid nitrogen - a commonly available coolant. Its actual molecular formula is YBa2Cu3O7, making it a "1-2-3" superconductor. YBCO compounds exhibit d-wave electron pairing. The patent for YBCO is held by Lucent Technologies. (You can view a list of the best-performing 1-2-3 compounds on the "Type 2" page.)
![]()
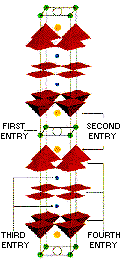 |
With the discovery of the ceramic
superconductors, came a need for a classification system to describe structure
types. The cuprate superconductors all have blocks of conducting CuO2
planes, alternating with insulating, spacing and separating layers. This
makes possible a systematic Naming Scheme
that allows for identification and comparison. The scheme chosen uses four
numbers. The first denotes the number of insulating layers between
adjacent conducting blocks. The second represents the number of spacing
layers between identical CuO2 blocks. The third gives the
number of layers that separate adjacent CuO2 planes within
the conducting block. And, the fourth is the number of CuO2planes
within a conducting block. Using the TlBa2Ca2Cu3O9 molecule depicted at left as an example, there is 1 insulating TlO layer, 2 spacing BaO layers, 2 separating Ca layers, and 3 conducting CuO2 planes - making it a "1223" type.
|
To further complicate matters,
several of the more popular structures have names that do NOT follow the
4-number scheme. For example, the compound Y1Ba2Cu3O7
is often referred to by the 3-number name "123". This delineates the number
of metal atoms (the stoichiometry) - without regard to atom location -
within the structure. Using the 4-number scheme, it would be classified
a "1212".
[Last page rev: Feb. 2016]