
As if ceramic superconductors were not strange enough, even more mysterious superconducting systems have been discovered. One is based on compounds centered around the "Fullerene". The fullerene name comes from the late designer-author Buckminster Fuller. Fuller was the inventor of the geodesic dome, a structure with a soccer ball shape. The fullerene - also called a buckminsterfullerene or "buckyball" - exists on a molecular level when 60 carbon atoms join in a closed sphere. When doped with one or more alkali metals the fullerene becomes a "fulleride" and has produced Tc's ranging from 8 K for Na2Rb0.5Cs0.5C60 up to 40 K for Cs3C60. In 1993 researchers at the State University of New York at Buffalo reported Tc's between 60 K and 70 K for C-60 doped with the interhalogen compound ICl.
Fullerenes, like ceramic superconductors, are a fairly recent discovery. In 1985, professors Robert F. Curl, Jr. and Richard E. Smalley of Rice University in Houston and Professor Sir Harold W. Kroto of the University of Sussex in Brighton, England, accidentally stumbled upon them. The discovery of superconducting alkali metal fullerides came in 1991 when Robert Haddon and Bell Labs announced that K3C60 had been found to superconduct at 18 K.
Larger, non-spherical pure carbon fullerenes that will superconduct have only recently been discovered. In April of 2001, Chinese researchers at Hong Kong University found 1-dimensional superconductivity in single-walled carbon nanotubes at around 15 Kelvin. And in February 2006, Physicists in Japan showed non-aligned, multi-walled carbon nanotubes were superconductive at temperatures as high as 12 K. Silicon-based fullerides like Na2Ba6Si46 will also superconduct. However, they are structured as infinite networks, rather than discrete molecules. Fullerenes are technically part of a larger family of organic conductors which are described below.
![]()
(TMTSF)2PF6
The first organic superconductor discovered.
Courtesy Laboratoire de Physique des Solides
"Organic" superconductors are part of the organic conductor family which includes: molecular salts, polymers and pure carbon systems (including carbon nanotubes and C60 compounds). The molecular salts within this family are large organic molecules that exhibit superconductive properties at very low temperatures. For this reason they are often referred to as "molecular" superconductors. Their existence was theorized in 1964 by Bill Little of Stanford University. But the first organic superconductor (TMTSF)2PF6 was not actually synthesized until 1980 by Danish researcher Klaus Bechgaard of the University of Copenhagen and French team members D. Jerome, A. Mazaud, and M. Ribault. About 50 organic superconductors have since been found with Tc's extending from 0.4 K to near 12 K (at ambient pressure). Since these Tc's are in the range of Type 1 superconductors, engineers have yet to find a practical application for them. However, their rather unusual properties have made them the focus of intense research. These properties include giant magnetoresistance, rapid oscillations, quantum hall effect, and more (similar to the behavior of InAs and InSb). In early 1997, it was, in fact (TMTSF)2PF6 that a research team at SUNY discovered could resist "quenching" up to a magnetic field strength of 6 tesla. Ordinarily, magnetic fields a fraction as strong will completely kill superconductivity in a material.
Organic superconductors are composed of an electron donor (the planar organic molecule) and an electron acceptor (a non-organic anion). Below are a few more examples of organic superconductors.
NOTE: For more on organics, click here.
![]()
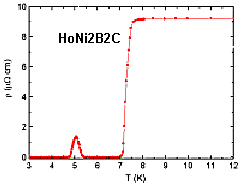
Discovered in 1993 by Bob Cava (currently at Princeton University) and Bell Labs, "Borocarbides" are one of the least-understood superconductor systems of all. It has always been assumed that superconductors cannot be formed from ferromagnetic transition metals - like iron, cobalt or nickel. It's the equivalent of trying to mix oil and water. However, in some borocarbides there is a "soap" that acts to bring these adversaries together. The crystallographic sites for the magnetic ions are thought to be isolated from the conduction path. This allows the cooper pairs to detour around the magnetic ions. Further, when combined with an element that has unusual magnetic properties - like holmium - "reentrant" behavior can also be in evidence in some borocarbides. Below Tc, where it should remain superconductive, there is a discordant temperature at which the material retreats to a "normal", non-superconductive state (see above graphic).
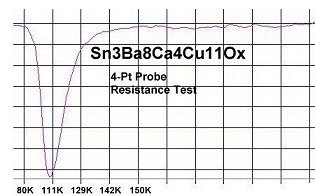
To date only one superconductor has been found that has zero resistance at a single temperature - the opposite of reentrant superconductivity. Click HERE to read more about this "resonant" superconductor.
Not only the borocarbides recede from a superconductive state at extreme low temperatures. In the compounds HoMo6S8 (Chevrel) and ErRh4B4 superconductivity suddenly disappears at around 1K. Click here to read more about this phenomenon. As can be seen from some of the below examples, the first metal site in the molecule is always occupied by a rare earth atom.
![]()
Flux lattices in UPt3
Courtesy Bell Labs
The "Heavy Fermions" sound like a family of overweight circus performers. But, they are yet another example of atypical superconductors. Heavy fermions are compounds containing rare-earth elements such as Ce or Yb, or actinide elements such as U. Their (inner shell) conduction electrons often have effective masses (known as quasiparticle masses) several hundred times as great as that of "normal" electrons, resulting in what's known as low "Fermi energy" (Ef). This makes them reluctant superconductors. Yet, at cryogenic temperatures, many of these materials are magnetically ordered, others show strong paramagnetic behavior, and some display superconductivity through a mechanism that quickly runs afoul of BCS theory. Research suggests cooper-pairing in the heavy fermion systems arises from the magnetic interactions of the electron spins (D-wave, P-wave, S-wave), rather than by lattice vibrations.
The first observation of superconductivity in a heavy fermion system was made by E. Bucher, et al, in 1973 in the compound UBe13; but, at the time was attributed to precipitated uranium filaments. Superconductivity was not actually recognized, per se, in a heavy fermion compound until 1979 when Dr. Frank Steglich of the Max Planck Institute for Chemical Physics in Solids (Dresden, Germany) realized it was a bulk property in CeCu2Si2. Heavy fermion superconductivity has since been observed in over 20 Ce compounds.
In April 2003 a heavy-fermion compound unambiguously exhibited the so-called "FFLO" state, where magnetism and superconductivity have a beneficial coexistence. The compound CeCoIn5 confirmed a theoretical model first put forth in 1964 by Fulde, Ferrell, Larkin, and Ovchinnikov (FFLO). Below are some heavy fermion compounds that will superconduct, along with their Tc's. As can be seen, their transition temperatures are in the range of Type 1 superconductors, which severely limits their use in practical applications.
CeCoIn5 2.3 K (first confirmed FFLO compound)Note: UGe2 and URhGe2 exhibit simultaneous ferromagnetism and superconductivity. Read more about this phenomenon in the below section on Ruthenates.
![]()
Magnetic topography of Sr2RuO4
In the mid 1990's, it was discovered that copper-oxygen planes are not the only superconducting facilitators within the layered perovskites. In 1994 physicists at IBM Zurich and at Hiroshima University collaborated to study the atomic planes of ruthenium-oxygen due to their similarity to copper-oxygen planes. Yoshiteru Maeno and colleagues found that the compound Sr2RuO4 exhibited superconductivity at 1.5 K. While this is an extremely cold Tc for a superconducting perovskite, it revealed a new area of potential among what are known as "Ruthenates". Shortly after that SrRuO and SrYRuO6 were also found to superconduct at similarly low temperatures.
When the crystalline structure of some of these materials is broken apart, its surface becomes increasingly ferromagnetic at low temperatures. This phenomenon flies in the face of condensed matter theory. So much so, that researchers have characterized them as an analog to superfluid Helium-3. And, when copper is added to the mix, even stranger things happen. In June 1999 New Zealand researcher Dr. Jefferey Tallon and his German colleague Dr. Christian Bernard discovered a ruthenium-cuprate** whose bulk is both a superconductor and a magnet. Although it was not the first compound discovered that exhibits coexisting ferromagnetism and superconductivity, it's remarkably high Tc of 58 K makes it truly distinct in the world of superconductors. Unlike "normal" superconductors, this compound becomes diamagnetic at about one-half Tc.
[**RuSr2(Gd,Eu,Sm)Cu2O8 or any parenthetical element partially substituted by Y.]
NOTE: To learn more about this material, click here.
![]()
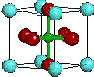
Ba0.6K0.4BiO3
Courtesy University of Texas
There are many phase transitions that matter goes through on its way to another state (e.g. ice changing to water requires a sudden increase in heat energy). Among the superconductors this is also the case at Tc, Hc and Jc. However, a superconductor has been discovered that exhibits no measurable change in its specific heat (the amount of energy required to increase its temperature by one degree) while going through up to 3 different "critical" magnetic fields. Ba0.6K0.4BiO3 seems to be the first material discovered that enters a "fourth order" phase transition (according to Ehrenfest's phase transition classification scheme) - something that has never before been observed in nature. Roy Goodrich of Louisiana State University and Donovan Hall of the National High Magnetic Field Laboratory announced this discovery in May, 1999. Not only is this an amazing anomaly in the field of superconductivity. It suggests that even higher order phases may exist in nature.
![]()
While no one can predict what future discoveries will be made in the field of superconductivity, some recent developments in the tungsten-bronze system suggest a new vista may be emerging. In July of 1999 researchers Y. Tsabba and S. Reich of the Weizmann Institute in Israel reported possible superconductivity near 91 K in the sodium-doped tungsten-bronze Na0.05WO3. This would be the first known HTS that is not a cuprate. Most tungsten-bronze compounds that are known to superconduct have Tc's below 4K - making this a truly tantalizing find. To learn more, click here.
Other categories of materials that theory suggests may produce superconductors are the higher silver fluorides and complex fluorides -- known as fluoroargentates. Fluoroargentates bear a strong similarity to oxocuprates, compounds that currently have the highest transition temperatures of all known superconductors. In October 2003 researchers Wojciech Grochala, Adrian Porch and Peter P. Edwards reported sudden drops in magnetic susceptibility within a large number of samples of Be-Ag-F. They attribute this to possible spherical regions of superconductivity - with a Tc up to 64 K - couched inside a ferromagnetic host. For more on this, click HERE.
With few exceptions (e.g. polysulphur-nitrides), most polymers resist being coaxed into a superconductive state. However, some organic polymers exhibit electrical resistance many orders of magnitude lower than the best metallic conductors. And, they do this at room temperature! These ultraconductors™, materials such as oxidized atactic polypropylene (OAPP), do not have zero resistance. But, their enhanced conductivity at ambient temperatures and pressures may actually allow them to compete with superconductors in certain fields. Polypropylene, for example, is normally an insulator. In 1985, however, researchers at the Russian Academy of Sciences discovered that as an oxidized thin-film, polypropylene can have a conductivity 105 to 106 higher than the best refined metals. The Meissner effect - the classic criterion for superconductivity - cannot be observed, as the critical transition temperature appears to be above the point at which the polymer breaks down (>700K). However, strong (giant) diamagnetism has been confirmed. A primer on ultraconductors™ is available by clicking here.
[Last page rev: Jan. 2007]
![]()