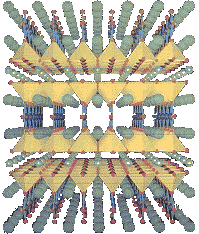
A Type 2 Layered Cuprate
Type 2 Superconductors

Except for the elements vanadium, technetium and niobium, the Type 2 category of superconductors is comprised of metallic compounds and alloys. The recently-discovered superconducting "perovskites" (metal-oxide ceramics that normally have a ratio of 2 metal atoms to every 3 oxygen atoms) belong to this Type 2 group. They achieve higher Tc's than Type 1 superconductors by a mechanism that is still not completely understood. Conventional wisdom holds that it relates to the planar layering within the crystalline structure (see above graphic). Although, other recent research suggests the holes of hypocharged oxygen in the charge reservoirs are responsible. (Holes are positively-charged vacancies within the lattice.) The superconducting cuprates (copper-oxides) have achieved astonishingly high Tc's when you consider that by 1985 known Tc's had only reached 23 Kelvin. To date, the highest Tc attained at ambient pressure for a material that will form stoichiometrically (by direct mixing) has been 147 Kelvin. And the highest Tc overall is 216 Celsius for a material which does not form stoichiometrically (see below list). It is almost certain that other, more-synergistic compounds still await discovery among the high-temperature superconductors.
The first superconducting Type 2 compound, an alloy of lead and bismuth, was fabricated in 1930 by W. de Haas and J. Voogd. But, was not recognized as such until later, after the Meissner effect had been discovered. This new category of superconductors was identified by L.V. Shubnikov at the Kharkov Institute of Science and Technology in the Ukraine in 1936(1) when he found two distinct critical magnetic fields (known as Hc1 and Hc2) in PbTl2. The first of the oxide superconductors was created in 1973 by DuPont researcher Art Sleight when Ba(Pb,Bi)O3 was found to have a Tc of 13K. The superconducting oxocuprates followed in 1986.
Type 2 superconductors - also known as the "hard" superconductors - differ from Type 1 in that their transition from a normal to a superconducting state is gradual across a region of "mixed state" behavior. Since a Type 2 will allow some penetration by an external magnetic field into its surface, this creates some rather novel mesoscopic phenomena like superconducting "stripes" and "flux-lattice vortices". While there are far too many to list in totality, some of the more interesting Type 2 superconductors are listed below by similarity and with descending Tc's. Where available, the lattice structure of the system is also noted.
|
Sn12SbTe11Ba2V2Mg24O50+ (As a (Z+12)212 structure) Sn11SbTe10Ba2V2Mg22O46+ (As a (Z+8)212 structure) Sn11SbTe10Ba2VMg23O46+ (As a (Z+8)212 structure) Sn10SbTe9Ba2MnCu21O42+ (As a (Z+4)212 structure) Sn9SbTe8Ba2MnCu19O38+ (As a Z212 structure) Sn8SbTe7Ba2MnCu17O34+ (As a V212 structure) Sn7Te7Ba2MnCu15O30+ (As an R212 structure) Sn7SbTe6Ba2MnCu15O30+ (As an R212 structure) Sn10SbTe4Ba2MnCu16O32+ (As a T212 structure) Sn9SbTe4Ba2MnCu15O30+ (As an R212 structure) Sn8SbTe4Ba2MnCu14O28+ (As a P212 structure) Sn9SbTe3Ba2MnCu14O28+ (As a P212 structure) Sn9Te3Ba2MnCu13O26+ (As an N212 structure) Sn6Sb6Ba2MnCu13O26+ (As an N212 structure) Sn5Te5Ba2VMg11O22+ (As a J212 structure) Sn5Sb5Ba2MnCu11O22+ (As a J212 structure) Sn4Te4Ba2MnMg9O18+ (As an F212 structure) Tl7Sn2Ba2MnCu10O20+ (As an H212 structure) Tl7Sn2Ba2TiCu10O20+ (As an H212 structure) Tl6Sn2Ba2TiCu9O18+ (As an F212 structure) Tl7Sn2Ba2SiCu10O20+ (As an H212 structure) Tl6Ba4SiCu9O18+ (As an F212 structure) Tl5Ba4SiCu8O16+ (As a D212 structure) (Tl5Sn2)Ba2SiCu8O16+ (As a D212 structure) (Tl5Pb2)Ba2SiCu8O16+ (As a D212 structure) (Tl5Pb2)Ba2Si2.5Cu8.5O17+ (As a D223 structure) (Tl5Pb2)Ba2Mg2.5Cu8.5O17+ (As a D223 structure) (Tl5Pb2)Ba2Mg2Cu9O18+ (As a D223 structure) (Tl5Pb2)Ba2MgCu10O20+ (As a D223 structure) (Tl4Pb)Ba2MgCu8O13+ (As a 9223 structure) (Tl4Ba)Ba2MgCu8O13+ (As a 9223 structure) (Tl4Ba)Ba2Mg2Cu7O13+ (As a 9223 structure) (Tl4Ba)Ba2Ca2Cu7O13+ (As a 9223 structure) (Tl4Ba)Ba4Ca2Cu10Oy (As a 9212/2212C intergrowth.) Tl5Ba4Ca2Cu10Oy (As a 9212/2212C intergrowth.) (Sn5In)Ba4Ca2Cu11Oy (As a B212/2212C intergrowth.) (Sn5In)Ba4Ca2Cu10Oy (As a B212/1212C intergrowth.) Sn6Ba4Ca2Cu10Oy (As a B212/1212C intergrowth.) (Sn1.0Pb0.5In0.5)Ba4Tm6Cu8O22+ (As a 1256/1212 intergrowth.) (Sn1.0Pb0.5In0.5)Ba4Tm5Cu7O20+ (As a 1245/1212 intergrowth.) (Sn1.0Pb0.5In0.5)Ba4Tm4Cu6O18+ (As a 1234/1212 intergrowth) Sn3Ba4Ca2Cu7Oy (As a 5212/1212C intergrowth.) |
+216 C +209 C +202 C +187 C +178 C +167 C +158 C +155 C +141 C +136 C +129 C +121 C +119 C +110 C +109 C +95 C +87 C +77 C +65 C +56 C +53 C +48 C +44 C +42 C +38 C +35 C +30 C +28 C +18 C +3 C 265 K 258 K 254 K 242 K 233 K 218 K 212 K 200 K 195 K 185 K 163 K 160 K |
 |
|
(Hg0.8Tl0.2)Ba2Ca2Cu3O8.33* HgBa2Ca2Cu3O8 HgBa2Ca3Cu4O10+ HgBa2(Ca1-xSrx)Cu2O6+ HgBa2CuO4+ |
138 K 133-135 K 125-126 K 123-125 K 94-98 K |
|
![]()
|
Tl2Ba2TeCu3O8+ Tl2Ba2YCu2O6 Tl2Ba2Ca2Cu3O10 (Tl1.6Hg0.4)Ba2Ca2Cu3O10+ TlBa2Ca2Cu3O9+ (TlSn)Ba4TmCaCu4O14+ (Tl0.5Pb0.5)Sr2Ca2Cu3O9 Tl2Ba2CaCu2O6 TlBa2Ca3Cu4O11 (Tl0.5Pb0.5Sn)Ba4Tm3Cu5O16+ TlBa2CaCu2O7+ Tl2Ba2CuO6 TlSnBa4Y2Cu4Ox |
147 K (Superconductors.ORG - 2016) 139 K (Superconductors.ORG - 2016) 127-128 K 126 K 123 K 121 K (Superconductors.ORG - 2005) 118-120 K 118 K 112 K 105 K (Superconductors.ORG - 2011) 103 K 95 K 86 K (Superconductors.ORG - 2007) |
![]()
|
SnSbBa2(Tm0.5Ca0.5)Cu3O8+ Sn4Ba4(Tm2Ca)Cu7Ox Sn2Ba2(Tm0.5Ca0.5)Cu3O8+ SnInBa4Tm3Cu5Ox Sn3Ba4Tm3Cu6Ox Sn3Ba8Ca4Cu11Ox SnBa4Y2Cu5Ox Sn4Ba4Tm2YCu7Ox Sn4Ba4TmCaCu4Ox Sn4Ba4Tm3Cu7Ox Sn2Ba2(Y0.5Tm0.5)Cu3O8+ Sn3Ba4Y2Cu5Ox SnInBa4Tm4Cu6Ox Sn2Ba2(Sr0.5Y0.5)Cu3O8 Sn4Ba4Y3Cu7Ox |
~147 K (Superconductors.ORG - 2025) ~127 K (TmTm-Ca structure only) ~115 K (Superconductors.ORG - 2005) ~113 K (Superconductors.ORG - 2005) 109 K (Superconductors.ORG - 2007) 109 K (One-of-a-Kind Resonant - 2006) 107 K (Superconductors.ORG - 2007) ~104 K (First Hi-Tc Reentrant - 2007) ~100 K (Superconductors.ORG - 2007) ~98 K (Superconductors.ORG - 2006) ~96 K (Superconductors.ORG - 2007) ~91 K (Superconductors.ORG - 2006) 87 K (Superconductors.ORG - 2005) 86 K (Aleksandrov, et al - 1989) ~80 K (Superconductors.ORG - 2005) |
![]()
|
Bi2Sr2TeCu3O8
Bi1.6Pb0.6Sr2Ca2Sb0.1Cu3Ox*** Bi2Sr2Ca2Cu3O10*** Bi2Sr2CaCu2O9*** Bi2Sr2(Ca0.8Y0.2)Cu2O8 Bi2Sr2CaCu2O8 BiSnBa4TmCaCu4O14 |
139K (Superconductors.ORG - 2016)
115 K (thick film on MgO substrate) 108-110 K 95 K 95-96K 91-92K 83K (Superconductors.ORG - 2012) |
*** Though not always listed as a component, a small amount of Lead (x=.2-.26) is often used with Bismuth compounds to help facilitate a higher-Tc crystalline phase. The PbII hole-dopes the cation layers, as well as dampens lattice vibrations.
![]()
*** The above compounds are all "infinite layer".
![]()
|
Pb3MgO5 Pb3Sr4Ca3Cu6Ox Pb3Sr4Ca2Cu5O15+ (Pb1.5Sn1.5)Sr4Ca2Cu5O15+ Pb2Sr2(Ca, Y)Cu3O8 |
307 K (Superconductors.ORG - 2016) 106 K (Superconductors.ORG - 2007) 101 K (Superconductors.ORG - 2005) ~95 K (Superconductors.ORG - 2006) 70 K (Cava, et al - 1989) |
![]()
|
AuBa2Ca3Cu4O11 AuBa2(Y, Ca)Cu2O7 AuBa2Ca2Cu3O9 |
99 K (Kopnin, et al - 2001) 82 K 30 K |
![]()
|
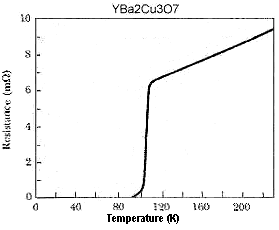
CdNbBa9Cu10O20+ (F212C structure) TiBa9Cu10O20+ (F212C structure) VBa9Cu10O20+ (F212C structure) ZrBa9Cu10O20+ (F212C structure) NbBa9Cu10O20+ (F212C structure) TeBa10Cu11O22+ (H212C structure) TaBa9Cu10O20+ (F212C structure) Y2Ba10Cu12O25+ (H212C structure) TiBa7Cu8Ox (B212C structure) TaBa7Cu8Ox (B212C structure) TeBa7Cu8Ox (B212C structure) YBa3Cu4Ox (9223C structure) YCaBa3Cu5O11+ (Y0.5Lu0.5)Ba2Cu3O7 (Y0.5Tm0.5)Ba2Cu3O7 Y3Ba5Cu8O18+ Y2Ba5Cu8O17+ YBa2Cu2MgO7+ TeCaBa4Cu6O16+ Y3CaBa4Cu8O18+ TeBa3Cu4Ox (Y0.5Gd0.5)Ba2Cu3O7 Y2CaBa4Cu7O16 Y3Ba4Cu7O16 Y2Ba5Cu7Ox NdBa2Cu3O7 Y2Ba4Cu7O15 GdBa2Cu3O7 YBa2Mg3Ox YBa2Cu3O7 TmBa2Cu3O7 YbBa2Cu3O7 YSr2Cu3O7 Lattice: TET |
337 K (Superconductors.ORG - 2016) 331 K (Superconductors.ORG - 2015) 327 K (Superconductors.ORG - 2015) 322 K (Superconductors.ORG - 2015) 314 K (Superconductors.ORG - 2015) 313 K (Superconductors.ORG - 2015) 313 K (Superconductors.ORG - 2015) 307 K (Superconductors.ORG - 2015) 273 K (Superconductors.ORG - 2017) 255 K (Superconductors.ORG - 2013) 255 K (Superconductors.ORG - 2014) 177 K (Superconductors.ORG - 2009) 107 K (Superconductors.ORG - 2010) 106 K (Superconductors.ORG - 2005) 104 K (Superconductors.ORG - 2005) 104 K (Superconductors.ORG - 2008) 104 K (Superconductors.ORG - 2012) 100 K (Superconductors.ORG - 2023) 99 K (Superconductors.ORG - 2014) 99 K (Superconductors.ORG - 2010) 98 K (Superconductors.ORG - 2013) 97 K (Superconductors.ORG - 2005) 96 K (Superconductors.ORG - 2006) 96 K (Superconductors.ORG - 2005) 96 K (Superconductors.ORG - 2008) 96 K 95 K 94 K 92 K (Superconductors.ORG - 2016) 92 K (See above graphic) 90 K 89 K 62 K |
![]()
|
GaSr2(Ca0.5Tm0.5)Cu2O7 Ga2Sr4Y2CaCu5Ox Ga2Sr4Tm2CaCu5Ox La2Ba2CaCu5O9+ (Sr,Ca)5Cu4O10 GaSr2(Ca, Y)Cu2O7 (In0.3Pb0.7)Sr2(Ca0.8Y0.2)Cu2Ox (La,Sr,Ca)3Cu2O6 La2CaCu2O6+ (Eu,Ce)2(Ba,Eu)2Cu3O10+ (La1.85Sr0.15)CuO4 SrNdCuO**** (La,Ba)2CuO4 (Nd,Sr,Ce)2CuO4 Pb2(Sr,La)2Cu2O6 (La1.85Ba.15)CuO4 |
99 K (Superconductors.ORG - 2006) 85 K (Superconductors.ORG - 2006) 81 K (Superconductors.ORG - 2006) 79 K (Saurashtra Univ., Rajkot, India - 2002) 70 K 70 K 60 K 58 K 45 K 43 K 40 K 40 K 35-38 K 35 K 32 K 30 K (First HTS ceramic SC discovered - 1986) |
**** First ceramic superconductor discovered without a non-superconducting oxide layer.
Comment: All of the above are copper perovskites, even though their metal-to-oxygen ratios are not exactly 2-to-3.
![]()
|
FeSe (monolayer) GdFeAsO1-x (Ca,Sr,Ba)Fe2As2 LiFeAs |
65 K 53.5 K 38 K 18 K |
Comment: The above are members of the newly-discovered iron pnictide family.
|
MgB2 Ba0.6K0.4BiO3 |
39 K (one of the highest known transition temperatures of any BCS superconductor) 30 K (First 4th order phase compound) |
![]()
| Nb3Ge
Nb3Si Nb3Sn Nb3Al V3Si Ta3Pb V3Ga Nb3Ga V3In |
23.2 K
19 K 18.1 K 18 K 17.1 K 17 K 16.8 K 14.5 K 13.9 K |
Comment: Among the binary alloys, these are some of the best performers; combining
Group 5B metals in a ratio of 3-to-1 with 4A or 3A elements.
| PuCoGa5 | 18.5 K (First SC transuranic compound) |
| NbN | 16.1 K |
|
Nb0.6Ti0.4 MgCNi3 |
9.8 K (First superconductive wire) 7-8 K (First all-metal perovskite superconductor) |
![]()
|
C
Nb Tc V |
15 K (as highly-aligned, single-walled nanotubes)
9.25 K 7.80 K 5.40 K |
![]()
|
RuSr2(Gd,Eu,Sm)Cu2O8
EuFe2(As0.79P0.21)2 ErNi2B2C YbPd2Sn UGe2 URhGe2 AuIn3 |
~58 K (Ruthenium-oxocuprate)
24 K (Ferromagnetic below 18K) 10.5 K (Nickel-Borocarbide) ~2.5 K (Heusler compound) ~1K (Heavy fermion) ~1K ( " ) 50 uK |
Comment: The above 7 compounds are all rare ferromagnetic
superconductors.
| Sr.08WO3
Tl.30WO3 Rb.27-.29WO3 |
2-4 K
(Tungsten-bronze)
2.0-2.14 K (") 1.98 K (") |
![]()
| SrTiO3 | 0.35 K |
![]()
(1.) "History of Physics Research in Ukraine", by Oleksandr Bakai and Yurij Raniuk, Kharkov Institute of Science and Technology, 1993.
Author's Comment: The Tc's noted on this page were obtained from
a variety of sources including, but not limited to, the CRC Handbook of Chemistry
and Physics, the N.I.S.T. database, Physica C, industry news sources,
and various private researchers. In cases where there was a discrepancy between
sources, the higher Tc or a range of Tc's has been listed.
[Last page rev: Jan. 2023]
![]()