


|
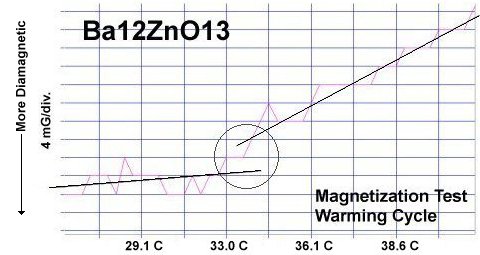
Though the atomic structures of BaO and ZnO (shown at left) are completely different, when they are blended together oxygen gets sandwiched between disparate-weight metals, heterodyning lattice vibrations of different frequencies. This results in periodic compression of the oxygen site and a "virtual redox", facilitating superconductivity. When the planar weight ratio is very high - at least 23 to 1 - room temperature superconductivity can result. 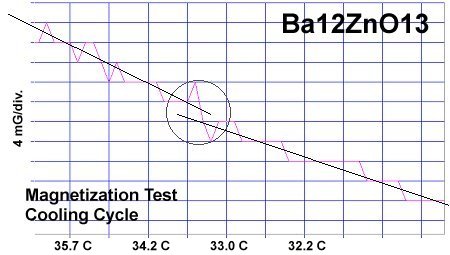
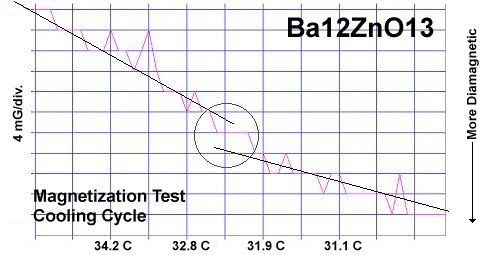
It is theorized that, when divalent metals of different atomic weights are positioned on opposite sides of an oxygen atom, periodic compression from lattice vibrations forces a momentary valency shift, causing a hole to appear at the oxygen site. That positively charged oxygen ion then acts as a mediator, encouraging electrons to pair up and produce a superconductive state, as shown in the below animation. The two red dots are paired electrons migrating as the region of positive charge moves. The reason the volume fraction (VF) is low in this RT superconductor is that, in the real world, parallel columns do not form identically. In one column the arrangement is 12 bariums then 1 zinc. But the column next to it might arrange as 8 bariums then 1 zinc then 4 bariums. This results in lattice vibrations being out-of-phase axially, suppressing superconductivity near that unit cell. In order to make a high VF room temperature superconductor, the structure must be homogeneous both chemically and structurally. This is why room temperature superconductors are not yet being mass produced. The level of homogeneity required is beyond current technology. |

The chemical precursors were pelletized at 60,000 PSI and calcined for one hour at 800C. Then sintering continued for 9 hours at 880C. Lastly, the pellet was annealed for 9+ hours at 500C in flowing O2. Testing temperatures were determined using an Omega type "T" thermocouple and precision OP77 DC amplifier. The magnetometer employed a Honeywell SS94A1F Hall-effect sensor with a sensitivity of 25 mv/Gauss.
RESEARCH NOTES: The blended oxides can be strongly hygroscopic. All tests should be performed immediately after annealing.
RE-PUBLICATION NOTICE: Elsevier Publishing, dba Elsevier Science, as well as Morris Communications, both print and broadcast divisions, are specifically prohibited from re-publishing any part of this news story.
E. Joe Eck
© 2017 Superconductors.ORG
All rights reserved.
 BACK to "News" page at Superconductors.ORG
BACK to "News" page at Superconductors.ORG