

In an effort to find support for a new theory of High-Temperature Superconductivity (HTSC) in the copper oxides, a search was conducted for an element with properties similar to copper. Magnesium was found to have identical +2 and +1 oxidation states[1]. And magnesium has an effective ionic radius that's within 2% of copper's EIR. Several known copper-oxide superconductors were then reformulated to place magnesium into the copper atomic sites. The resulting materials appeared to be a direct analog to the copper-oxide family.
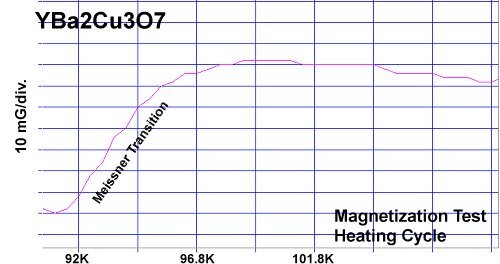
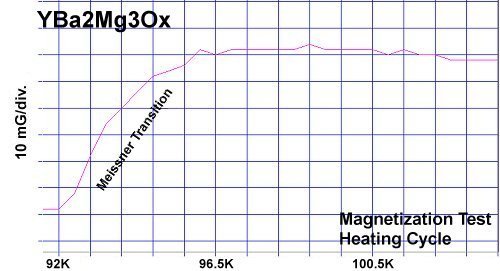
At page top are magnetization plots of YBCO and the new YBMO formulation. A commercial-grade YBCO sample[2] was obtained and ground into powder. Then a YBMO sample was synthesized and powdered. Both samples were tested in the same magnetometer under identical conditions. Over the course of five magnetization tests the critical transition temperature (Tc) of YBMO was found to be within a fraction of a degree of YBCO. And its diamagnetic transition was about 86% as strong. The weaker diamagnetic transitions of the YBMO sample were likely due to less powder in the gel cap. The weight of a YBMO molecule is 79% of a YBCO molecule. But the YBMO capsule weighed just 63% of the YBCO capsule.
|
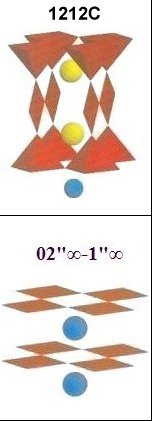
Magnesium was also inserted into the copper sites of two different "infinite layer" compounds. Though infinite layer superconductors form with a low volume fraction, they can serve to confirm that magnesium has the same functionality in a layered 02"∞-1"∞ structure as it does in the 1212C structure of YBCO (structures shown at left). The compound SrCaCu2O4 has an established Tc of 110 Kelvin. In the below test plot reformulated SrCaMg2Ox[3] shows a Tc just above 111K. (The straight lines drawn through the noise approximate the average of the data points, skewing apart at Tc.) 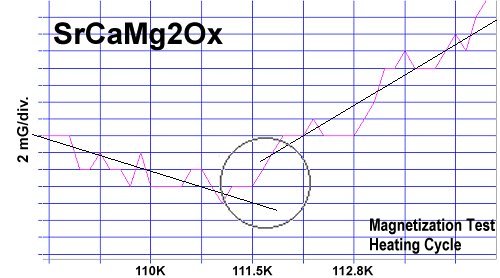
|
The below plots show the infinite layer compound CdCaCu2O4 beside its magnesium analog CdCaMg2Ox. Again the transition temperatures are about one degree apart and the volume fraction is lower.
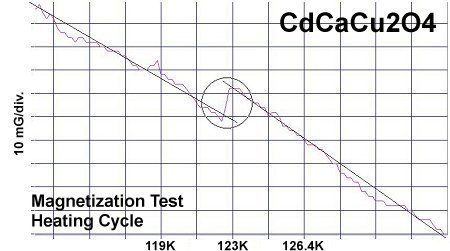
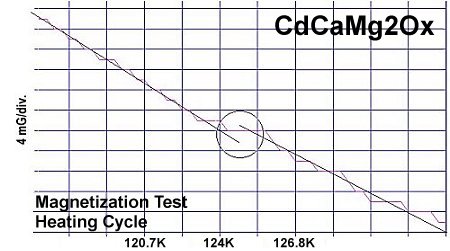
The theory of high-temperature superconductivity[4] that led to this discovery postulates that the valency of Cu (and now Mg) is shifting back and forth from +2 to +1 in sync with periodic compression of adjacent oxygen atoms. This compression results from planar weight disparity (PWD) on opposite sides of the oxygen atoms. Experimental evidence has found that PWD correlates directly with Tc. This facilitates the formation of Cooper pairs and, ergo, momentary superconductivity. However, since magnesium-oxide is not antiferromagnetic (AF), the result is not a full-fledged superconductor. Cooper pairing, and thus superconductivity, are transient. There is no resonance - only a brief Meissner effect due to the pairing of electrons being random.
Stoichiometric ratios of the below chemicals were used in the synthesis of these materials:
Y2O3 99.99% (Alfa Aesar) The chemical precursors were pelletized at 60,000 PSI. If the mix contained CaCO3, the pellet was first calcined at 760C for one hour. Then the pellet was sintered for 8 hours at 880C and annealed for 10+ hours at 500C in flowing O2. The magnetometer employed twin Honeywell SS94A1F Hall-effect sensors with a tandem sensitivity of 50 mv/Gauss. Testing temperatures were determined using an Omega type "T" thermocouple and precision OP77 DC amplifier.
RESEARCH NOTES: These materials can be strongly hygroscopic. All tests should be performed immediately after annealing. Typically copper-oxide superconductors turn charcoal black after being sintered. The color after sintering of these magnesium superconductors was a bright white.
RE-PUBLICATION NOTICE: Elsevier Publishing, dba Elsevier Science, as well as Morris Communications, both print and broadcast divisions, are specifically prohibited from re-publishing any part of this news story.
E. Joe Eck
Patent Pending US 62/391,842
© 2016 Superconductors.ORG
All rights reserved
1. Low valent magnesium compounds with Mg(I) have been achieved using bulky ligands.
2. The YBCO test pellet was obtained from Colorado Superconductor, Fort Collins, CO
3. Although the oxygen content is believed to be the same with Mg as it was with Cu,
Ox denotes that this will need to be determined for certain through x-ray diffraction.
4. E. Joe Eck, Superconductors.ORG
 BACK to "News" page at Superconductors.ORG
BACK to "News" page at Superconductors.ORG