
Organic conductors are materials made of relatively large organic molecules, about 20 atoms each. Their history started in 1964 when Bill Little (Stanford U.) suggested that the critical temperature of superconductors could be increased and he applied his theory to a polymer chain.
Most materials composed of organic molecules are normally not metals
because of hybridization which leaves their conduction and valence bands
filled. This property was first overcome by combining planar organic
molecules with nonorganic anions (ClO4, PF6 etc.) which serve as
acceptors or donors thus resulting in the appearance of partially filled
conduction and/or valence bands. Such materials are called charge transfer
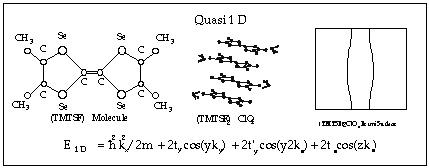
salts. In 1981 Klaus Bechgaard synthesized (TMTSF)2ClO4 (see diagram below),
the first organic
material that was superconducting at ambient pressure. Although, it has
a relatively low superconducting transition temperature (1.2 K), the
interest in superconductivity and other rather unusual properties
in organic materials exploded after this discovery.
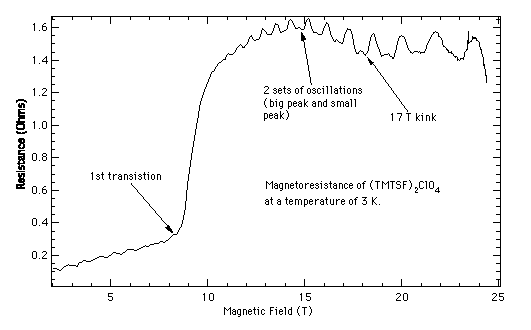
Since 1981, over 400 organic conductors have been synthesized, over 50 of which are superconducting. During this time the superconducting transition temperature in these materials has risen from 1.2 K to 12.6 K. Also in this time period, a number of other interesting electronic states have been observed in organic conductors including charge density waves (CDW), spin density waves (SDW), field induced spin density waves (FISDW, of course), the quantum Hall effect, and angular magnetoresistance oscillations. In addition, more traditional effects that are found in metals, such as the de Haas-van Alphen effect and the Shubnikov-de Haas effect, are greatly enhanced in organic conductors. The study of all these properties is enhanced by the determination of the electronic properties of these materials through the de Haas-van Alphen effect and the Shubnikov-de Haas effect. This has caused at least one theoretician to remark that "organic conductors are a laboratory of solid state physics."
Because organic conductors are complicated organic salts, they
have many free parameters that can be adjusted to carefully fine tune
their chemical structure. Consequently their electronic sucture can
also be easily adjusted and fine-tuned. In addition their electronic
structure is unique because they have low Fermi energies and are
electronically very clean, making it easy to study the intricacies of
their Fermi surfaces through the observation of quantum oscillations.
The low Fermi energy makes high magnetic field experiments more interesting
due to the impact of the magnetic energy on the Fermi surface structure.
As an example, Ef = 7.0 eV for Pb, whereas Ef for a typical organic is
approximately 0.01 eV (50 T ~ 0.003 eV). These
properties make them ideal for a number of solid state physics studies.
 BACK to the "Atypical" page at Superconductors.ORG
BACK to the "Atypical" page at Superconductors.ORG
 BACK to the "Terminology" page at Superconductors.ORG
BACK to the "Terminology" page at Superconductors.ORG